✎ 1 Atoms and Materials
✎ 1.1 Atomic Structure
✎
✎ National Curriculum Learning Objectives:
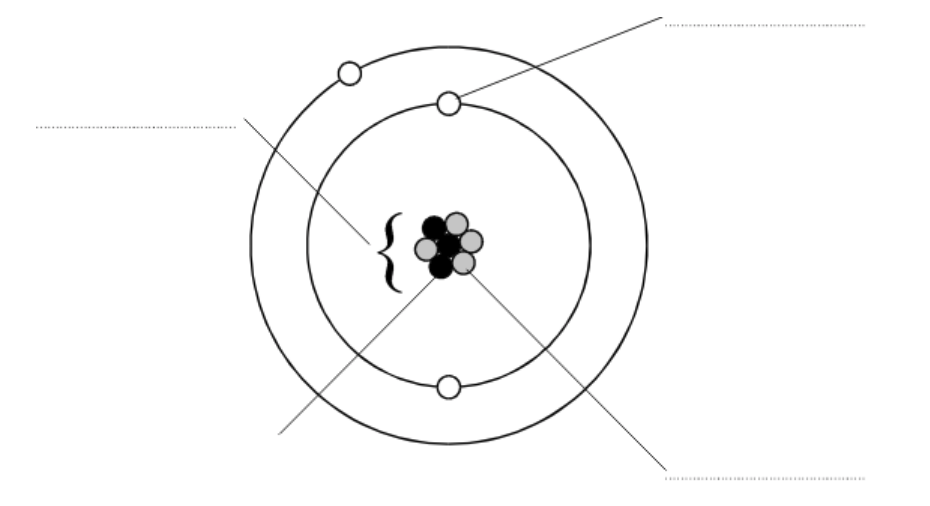
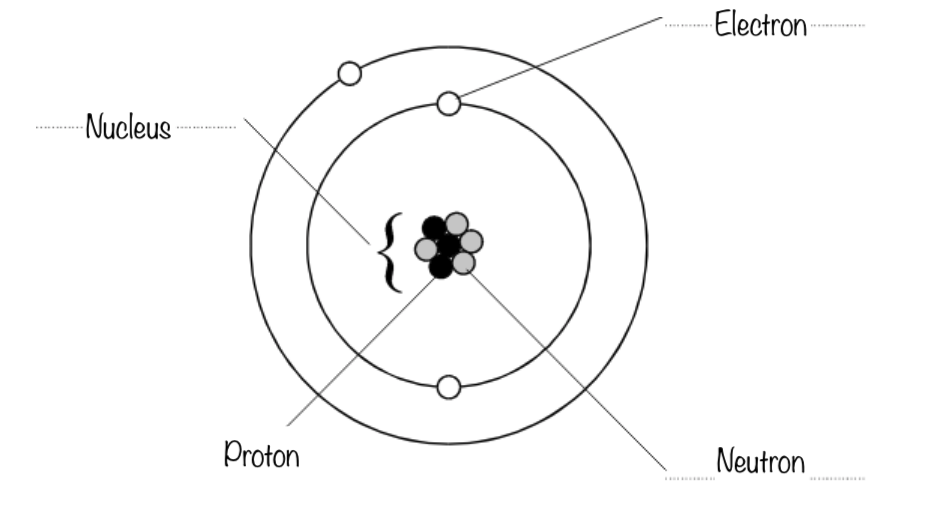
- Name the three types of particles present in an atom (protons, neutrons, electrons)
- Describe the properties of protons, neutrons and electrons in terms of mass and charge
- Use atomic number and mass number to determine the number of protons, neutrons and electrons in an atom
- Describe the electron arrangement/configuration for an atom of a given element
- Explain what isotopes are and how they differ for the same element
💬 Questions – discuss with your partner
- Describe the basic model of an atom
- What do the terms ‘atomic number’ and ‘mass number’ represent?